ar lewis dot structure|Lewis Dot Symbols and Lewis Structures (Writing Lewis Symbols for At : Bacolod Draw the Lewis dot structure of a given molecule or ion. Draw resonance structures of some molecules. Assign formal charge to an atom in a dot structure. . Login to Pinay Sex Scandal - Watch Porn Sex Videos Online | KANTOTIN
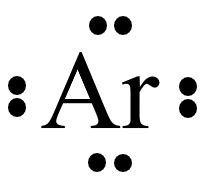
ar lewis dot structure,111. 23K views 10 years ago. A step-by-step explanation of how to draw the Lewis dot structure for Ar (Argon). I show you where Argon is on the periodic table and how to .
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that .
Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a .
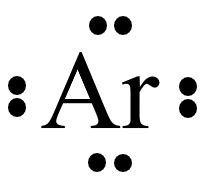
Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. These Lewis .ar lewis dot structureLewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. These Lewis . Draw the Lewis dot structure of a given molecule or ion. Draw resonance structures of some molecules. Assign formal charge to an atom in a dot structure. .THE EASY METHOD PROCEDURE TO DETERMINE A LEWIS DOT STRUCTURE. The H2O H 2 O Molecule. The OCl− O C l − Ion. The CH2O C H 2 O Molecule. Using Lewis .Learn how to draw Lewis dot diagrams of the elements using electron configuration and electron distribution into shells. See examples of electron distributions into shells for the first three periods and the .Lewis dot structures represent atoms with their atomic symbol surrounded by valence electrons, which are represented as dots. This type of symbolic representation can .A Lewis electron dot symbol (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of .
What are lewis structures? In 1916, American chemist, Gilbert N. Lewis, introduced bond lines to electron dot structures. These structures, also known as lewis structures or electron dot structures, are drawings .We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols: The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with .The former, known as a ‘Lewis dot diagram,’ indicates a pair of shared electrons between the atomic symbols, while the latter, known as a ‘Lewis structure,’ uses a dash to indicate the pair of shared electrons that form a covalent bond. More complicated molecules are depicted this way as well.Some old concepts such as Lewis dot structure and valency are still rather useful in our understanding of the chemical properties of atoms and molecules, and new concepts involving quantum mechanics of chemical bonding interpret modern observations very well. . [Ar]4s^2 \label{3}\] where the \([Ar]\) stands for the configuration of argon .We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of .Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
The argon (Ar) atom, a noble gas with atomic number 18, has a Lewis structure that reflects its full valence shell of eight electrons, making it chemically inert. It has an electron configuration of [Ne]3s²3p⁶, indicating a filled 3s and 3p subshell. This configuration results in a stable, octet-compliant structure, precluding bonding under .
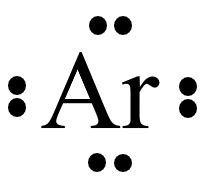
This page titled 4.1: Lewis Electron Dot Structures is shared under a CK-12 license and was authored, remixed, and/or curated by CK-12 Foundation via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. Ionic substances are completely held together by ionic bonds.Arkansas State University Department of Chemistry and Physics: Worksheets: Lewis Dot Structures. For each of the following, draw the Lewis Dot Structure, give the electron arrangement (E.A.) and the molecular geometry (M.G.): PF 5: CS 2: BrO 3- NH 4 + SCl 4: BrF 5 : BF 3: SCl 6: PH 3 : NF 3: SO 4 2-CO 3 2- SiCl 4: ClO 3-CH 2 O : NO 3-O 3:Lewis Dot Symbols and Lewis Structures (Writing Lewis Symbols for AtA Lewis electron dot symbol (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol . For the Al3+ structure use the periodic table to find the total number of valence electrons for Al. Once we know how many valence electrons there are in Alum.
Lewis structures, also known as electron dot structures, are named after Gilbert N. Lewis, who described them in a 1916 article titled, "The Atom and the Molecule." Lewis structures depict the bonds between atoms of a molecule, as well as any unbonded electron pairs. You can draw a Lewis dot structure for any covalent molecule or . Lewis structure is a simplified representation of all the valence electrons of an atom or a molecule. Valence electrons are the electrons present in the outermost shell of an atom and those that get involved in chemical bonding. Here is the list of all elements (1 to 118) in the periodic table with their Lewis dot structure also called electron .To use the Lewis Structure Calculator follow these steps: Enter the formula of the molecule in the field provided for it. For example, if we want to obtain the Lewis structure of the Sulfate ion, SO 4 – 2, we must first . For the K+ structure use the periodic table to find the total number of valence electrons for K. Once we know how many valence electrons there are in Potassi.
In a Lewis Structure, you draw a molecular structure that accounts for the valence electrons of all the participating atoms, showing the connectivity (bonds) as lines between atoms, and lone electrons as dots. In section 8.6 you will use this Lewis dot structure to predict the three dimensional geometry of a molecule.
Krypton (Kr), a noble gas with atomic number 36, has eight valence electrons. Its Lewis dot structure is represented by eight dots surrounding the symbol ‘Kr’, denoting a full outer shell. Krypton’s electron configuration is [Ar]3d¹⁰4s²4p⁶, reflecting its stable, inert nature due to a complete octet in its outermost shell.Some old concepts such as Lewis dot structure and valency are still rather useful in our understanding of the chemical properties of atoms and molecules, and new concepts involving quantum mechanics of chemical bonding interpret modern observations very well. . [Ar]4s^2 \label{3}\] where the \([Ar]\) stands for the configuration of argon .
The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. . Lewis dot symbols provide a simple rationalization of why elements form compounds with the observed stoichiometries. In the Lewis model, the number of bonds formed .
ar lewis dot structure|Lewis Dot Symbols and Lewis Structures (Writing Lewis Symbols for At
PH0 · Lewis Electron Dot Structures
PH1 · Lewis Dot Symbols and Lewis Structures (Writing Lewis Symbols for At
PH2 · Lewis Dot Symbols and Lewis Structures (Writing Lewis Symbols
PH3 · Lewis Dot Structures
PH4 · Lewis Dot Structure for Argon Atom (Ar)
PH5 · Lewis Dot Diagrams of the Elements
PH6 · 9.2: Lewis Electron Dot Diagrams
PH7 · 7.2 Lewis Dot Structures – Introduction to Chemistry
PH8 · 6.1 Lewis Electron Dot Symbols
PH9 · 4.3 Lewis Dot Structures